|
Total Electron |
Electron-Domains |
Bonding |
Nonbonding |
Molecular |
Hybridized Orbitals |
Associated Angles |
Example |
|---|---|---|---|---|---|---|---|
| 2 | Linear | 2 | 0 | 
|
sp | CO2 | |
| Linear | |||||||
| 3 | Trigonal Planar | 3 | 0 | 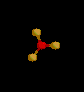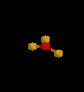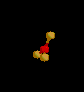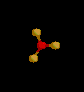
|
sp2 | BF3 | |
| Trigonal Planar | |||||||
| 2 | 1 | 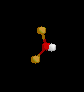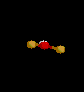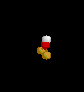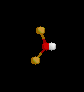
|
NO2- | ||||
| Bent | |||||||
| 4 | Tetrahedral | 4 | 0 | 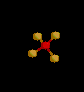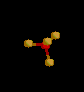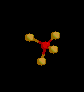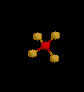
|
sp3 | CH4 | |
| Tetrahedral | |||||||
| 3 | 1 | 
|
NH3 | ||||
| Trigonal Pyramidal | |||||||
| 2 | 2 | 
|
H2O | ||||
| Bent | |||||||
| 5 | Trigonal Bipyramidal | 5 | 0 | 
|
sp3d | PCl5 | |
| Trigonal Bipyramidal | |||||||
| 4 | 1 | 
|
SF4 | ||||
| Seesaw | |||||||
| 3 | 2 | 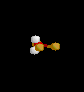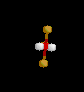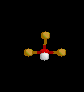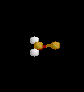
|
ClF3 | ||||
| T-shaped | |||||||
| 2 | 3 | 
|
XeF2 | ||||
| Linear | |||||||
| 6 | Octahedral | 6 | 0 | 
|
sp3d2 | SF6 | |
| Octahedral | |||||||
| 5 | 1 | 
|
BrF5 | ||||
| Square Pyramidal | |||||||
| 4 | 2 | 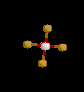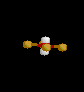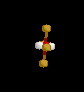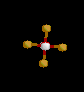
|
XeF4 | ||||
| Square Planar |